Agricultural Literacy Curriculum Matrix
Lesson Plan
FoodMASTER Middle: Fats and Oils
Grade Level
Purpose
Students will explore the fat content of commonly consumed foods, observe physical properties of lipids (margarine, butter, and vegetable oil) to distinguish between saturated and unsaturated fat, and observe the action of emulsifiers in heterogeneous and homogenous mixtures. Grades 6-8
Estimated Time
Materials Needed
Engage:
- Fascinating Fats student handout, 1 per student (Key)
- Nutrition Fact labels from a fast food restaurant.
- These can be obtained on the restaurant website, the USDA's FoodData Central, or by using the example labels provided.
Lab 1:
Teacher Materials Demonstration
(see Suggested Instructional Plan step 6)
- Safety goggles
- Apron (optional)
- Kitchen timer or stopwatch
- 4 Small, clear plastic cups
- 1/2 Cup cold butter
- 1/2 Cup cold margarine (If possible, when purchasing margarine, choose a brand that contains the words “partially hydrogenated” within the ingredients list.
- 2 Hot plates or 1 double burner
- 2 Saucepans
- 2 Thermometers
- 2 Wooden spoons
Student Materials, per group of 4-5 students
- Fatty Distinction student handout, 1 per student (Key)
- Lipid Language lab sheet, 1 per student (Key)
- Safety goggles
- Apron (optional)
- 1 Small bowl with 2 cups of ice
- 1 Kitchen timer or stopwatch
- 2 Cups ice
- 1 Small, clear, plastic cup containing 1/4 cup assigned fat type
- 1 Thermometer
Lab 2:
Teacher Materials
- 12 plastic or Styrofoam cups
- 3 and 3/4 cups olive oil
- 2 Tbsp. honey
- 2 Tbsp. salt
- 3 and 3/4 cups white vinegar
- 2 Tbsp. ground mustard
- 2 Tbsp. paprika
- 3 Bags of romaine lettuce
- Napkins
- 1 Bottle dish detergent
- 1 Dishtowel
Student Materials, per group of 4-5 students
- Examining Emulsions lab sheet, 1 copy per student (Key)
- Safety goggles
- Aprons (optional)
- 5 Medium mason jars
- 5/8 Cups vinegar in plastic or Styrofoam cup
- 5/8 Cups olive oil in plastic or Styrofoam cup
- 1 Teaspoon of each: ground mustard, honey, paprika, and salt
- 1 Large bowl (diameter = 8” or larger)
- 1 measuring spoons
- 1 Liquid measuring cup (1 cup or larger)
- 1 Black marker
- 5 Sticky labels
- 1 Kitchen timer or stopwatch
- 6 Paper plates (any size)
- 1 Whisk
- 1/2 bag or 1 head of fresh romaine lettuce
- 6 Plastic forks
Optional Lab Extension
- emulsion droplet
- 1 microscope slide and cover slip
- 1 microscope
- 1 small cup of water
- 1 medicine dropper
Essential Files
Vocabulary
emulsion: a mixture of liquids in which small drops of one liquid are mixed throughout another liquid
heterogeneous mixtures: a solution in which one substance mixes with another but does not dissolve
homogenous mixtures: a solution in which one substance is completely dissolved in another
melting point: the temperature at which a solid changes to a liquid
saturated fat: a type of fat containing a high proportion of fatty acid molecules without double bonds, considered to be less healthy in the diet than unsaturated fat
solidification: the process by which a liquid changes into a solid
unsaturated fat: a type of fat containing a high proportion of fatty acid molecules with at least one double bond; considered to be healthier in the diet than saturated fat
Did You Know?
- Butter is about 80% fat. The rest is mostly water.1
- While butter is made from milk, margarine is made from vegetable oils.2
- Recent studies show that consuming full-fat dairy products have positive effects such as lowering the risk of diabetes and maintaining a healthy weight.3
Background Agricultural Connections
Types of Fats and Their Impact on Health
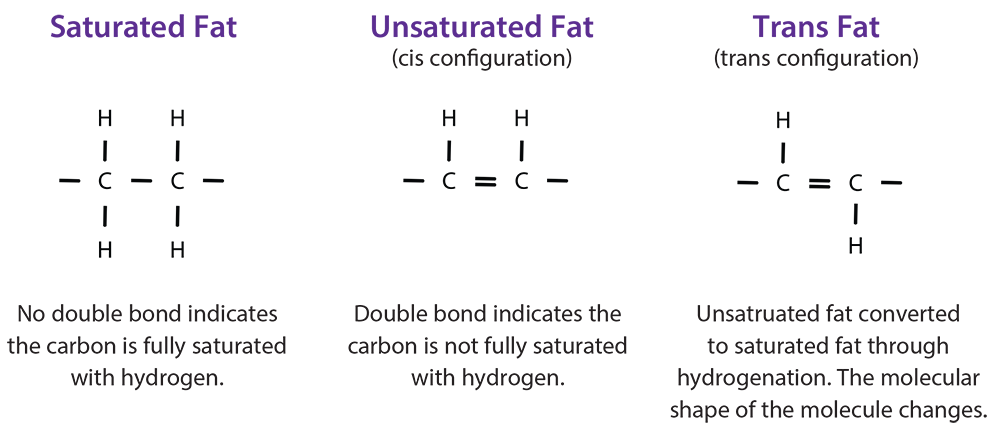
There are different types of fat including saturated, unsaturated, and trans (hydrogenated) fat. Unsaturated fats contain one or more double bonds. Saturated fats have no double bonds and are saturated with hydrogen. In excess, fats lead to many health problems like high blood pressure and stroke. Although trans fats can occur naturally in some products like butter, they are typically unsaturated fats that have been chemically altered (partially hydrogenated). When unsaturated fat is partially hydrogenated, a geometric isometric form of an unsaturated double bond is formed, resulting in trans fat. Margarine is an example of a partially hydrogenated fat. Because trans fats are typically not natural and are high in saturated fat, we should limit them in our diets. However, it is important to consume adequate amounts of fat in our diet because it is needed for energy, nerve and brain function, and growth. We should choose foods that are high in unsaturated fats, moderate in saturated fatty acids, and free of trans fats. Avoiding overconsumption of these foods will help decrease the risk for diet-related diseases such as heart disease, stroke, overweight/obesity, and Type 2 Diabetes.

Melting Points, Solidification, and Hydrogenation
Many students misunderstand chemical and physical changes. Students may think a change of state is chemical, when it is truly physical. You can use the concepts of melting point and solidification to correct these misconceptions in a hands-on, visual manner. Melting point is the point at which a solid fat turns to liquid. It varies from food to food. Solidification, or freezing, is the point at which a liquid turns to a solid. Butter melts at 90-95°F; however, there is no set point for butter to solidify because of its complexity. The exact fat/fatty acid composition is not the same from product to product. Butter will usually solidify around 60-65°F. The melting point of margarine is slightly higher than butter because of the presence of hydrogenated fats. It melts at 94-98 °F. Like butter, there is no set point at which it will solidify; it will usually solidify around 65-70°F. Solid fats have different melting points because they are not pure substances, but rather combinations of mixed triglycerides. Triglycerides are considered the major form of fat and is the storage form of fat in our bodies. Triglycerides are made up of 3 fatty acid chains attached to a glycerol backbone.
Fats in the form of oil also have melting points; however, they are much lower than fats that are solid at room temperature. The melting points of oils vary based on their fat content. For example, the melting point of olive oil is 21°F, but peanut oil is much higher at 28°F. In general, the lower the melting point, the healthier the oil. Based on this information, olive oil would be the better choice. Oils can be converted to solids through a process called hydrogenation, in which all or some (partial hydrogenation) are converted to saturated bonds by the addition of hydrogen. Hydrogenation is a chemical process in which hydrogen atoms are added to the unsaturated carbons, making them saturated. The process occurs only at very high temperatures and results in the production of trans fatty acids (cis converted to trans configuration) among remaining unsaturated bonds in partially hydrogenated oils. Foods higher in hydrogenated fats, or trans fats, have higher melting points. Some factors that influence melting points include the number of carbons in the molecule, the degree of unsaturation, and the molecular configuration (cis versus trans). Fats with longer carbon chains have higher melting points.
Emulsions, Fat Polarity, and Cell Membranes
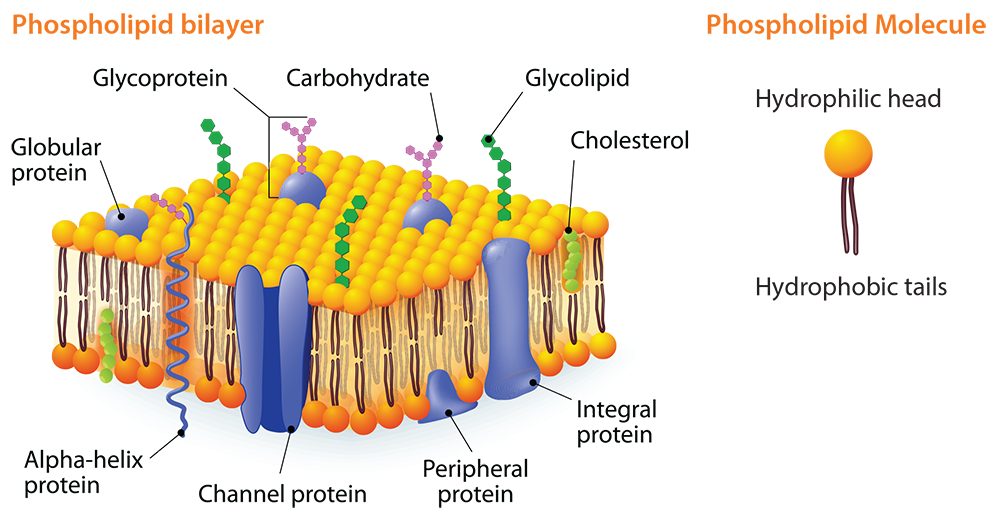
Heterogeneous mixtures have more than one color, substance, or texture visible. Homogeneous mixtures are the same throughout with a uniform appearance. An emulsion is a mixture of two immiscible liquids, like water and oil or vinegar and oil, where one is dispersed in small globules throughout the other. Immiscible liquids are not able to mix without an emulsifier that will create an emulsion. Emulsions are considered a heterogeneous mixture. Two liquids that are immiscible have opposite polarity. A liquid is either polar or non-polar. Polar liquids are miscible in other polar liquids and non-polar liquids are miscible in other non-polar liquids. In other words, miscible liquids will readily mix with each other without the help of an emulsifier. On the other hand, polar and non-polar liquids do not mix together without an emulsifier. When fats, typically oils, are mixed with an emulsifier, such as eggs, they form an emulsion.
The concept of fat and oil polarity can be used to teach students about the cell membrane. A major component of a cell’s membrane is phospholipids. A phospholipid molecule has one end that is hydrophilic (water-loving). The other end is hydrophobic (water-fearing). The cell membrane has two phospholipid layers known as the phospholipid bi-layer. The hydrophilic heads of one layer face outward toward the extracellular environment and, in the other layer, they face inward toward the cytoplasm. This double layer is semi-permeable and only certain types of molecules can diffuse across the membrane.
FoodMASTER Middle Lessons
FoodMASTER (Food, Math and Science Teaching Enhancement Resource) is a compilation of programs aimed at using food as a tool to teach mathematics and science. For more information see the Background & Introduction to FoodMASTER for Middle School. This lesson is one in a series of lessons designed for middle school:
Engage
- Ask students to raise their hands if they have eaten at a fast food restaurant at least one time in the last week. Ask those students to keep their hands raised if they have eaten fast food more than once in the last week. Begin a short class discussion about the nutritional benefits or drawbacks of eating fast foods and if it differs from home cooking.
- Give each student one copy of the Fascinating Fats student handout. Instruct students to read the first page and then complete the worksheet. Depending on available time, this activity can be completed in class or as homework. Note the following:
- Students can find Nutrition Facts for most popular fast food restaurants on their websites. If the student is unable to find the Nutrition Facts, they should be directed to USDA’s FoodData Central, or the labels provided.
- If students choose to use the provided food labels, see the attached teacher's key for answers to the Investigating Your Health lab questions. Answers to questions based on other food labels will vary.
- If completed in-class, allow students to work in small groups on the worksheet to further explore the topic and respond to questions.
- Follow-up with a class discussion about student findings related to sources of fat in their diets and student generated ideas for making healthier choices.
Explore and Explain
Lab 1: Lipid Language
Teacher Preparation:
- Review information found in the Background Agricultural Connections section of the lesson, lesson Procedures, and the attached Essential Files.
- Assign each student group 1 fat type (butter, margarine, or oil).
- Note that the teacher demonstration will show students the temperature and time it takes for two types of solid fat (i.e. butter, margarine) to melt. After melting the solid fats, you will divide each liquid fat evenly between two small, clear plastic cups.
- Distribute assigned liquid fats to student groups. Student groups will need to observe the liquid fats as part of the “cold treatment” procedure.
- Timesaver: Use the demonstration video in place of the in-class teacher-led demonstration. Provide students with the data below after your class has viewed the video.
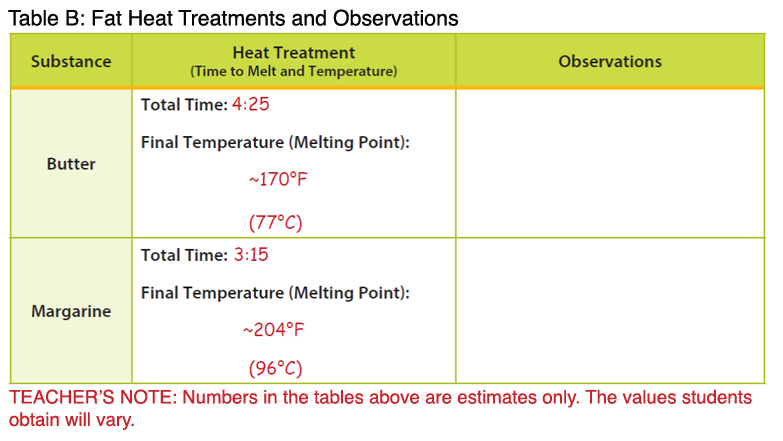
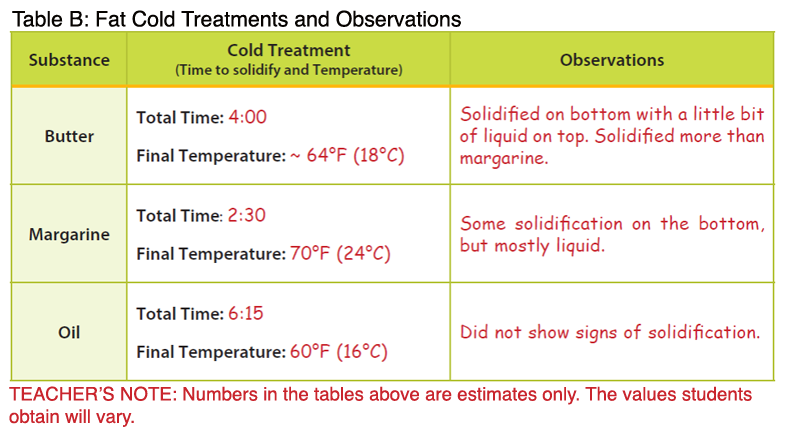
- Note: The temperatures below are higher than the melting points described in the background. The temperature of the melted butter and margarine were measured after the products had completely melted, resulting in an overall higher temperature.
Procedures:
- Consider having your students research the types of lipids (fat), their role in the body, their dietary sources, and the health benefits/consequences of consuming them prior to beginning the lab investigation.
- Distribute lab materials. It is recommended that materials are organized into stations for easier distribution. Students should be arranged in small groups of 4-5. Each group should receive the lab supplies outlined in the Materials section as well as 1 copy of the Lipid Language lab sheet and one copy of the Fatty Distinction student handout.
- Ask students to read Fatty Distinction and complete the focus questions for this lab investigation.
- Before beginning the lab investigation:
- Require students to wash their hands.
- DO NOT allow students to taste the lipids featured in this lesson. Students will be given the opportunity to taste a healthy fat (olive oil) in Lab 2: Lipid Language.
- Emphasize the importance of practicing good food safety behaviors by not consuming substances used as part of the lab investigation.
- Begin the student lab investigation by asking students to make a prediction about the rate different fat types melt and solidify. You may also choose to show the provided video lab demonstration, Food Explorations Lab I: Lipid Language. The video will help students understand the procedures and visual observations.
- Launch the teacher demonstration. The demonstration will show students how to find the melting points of butter and margarine. For an in-class demonstration use the following steps:
- Demonstrate for students how to find the melting points of butter and margarine. Vegetable oil will not undergo heat treatment because it is already a liquid at room temperature.
- Add 1/2 cup of cold butter to a saucepan and 1/2 cup of cold margarine to a second saucepan. You may choose to heat one fat type at a time or ask for student assistance. The solid fats may take slightly longer to melt due to the use of a double burner versus conventional stovetop.
- Once each solid fat is added to the saucepans, the stopwatch should be started to ensure accurate recording of the amount of time it takes each fat to turn into a liquid.
- Continue to heat each fat type on medium heat until it is completely melted. Once each fat has completely melted, the time and temperature should be taken.
- Record the time it took to melt each fat type in a place visible to students. Students should record this data in Table B under the column labeled “Heat Treatment.”
- Measure the temperature of each melted fat type and record the data in a place visible to students. Students should record this data in Table B under the column labeled “Heat Treatment.”
- Pour 1/4 cup of each melted fat type into a separate plastic cup to be used for student observation later in the lab investigation.
- After the teacher demonstration, students will complete the procedures for “cold treatment” with their assigned fat type. Once the fats have been submerged in ice, the solidification process takes approximately 10 minutes.
- Students should find the following results:
- Butter: Butter represents a saturated fat. During heat treatment, the butter will melt more slowly compared to margarine; however, it will be completely melted at a lower temperature. During cold treatment, the butter will solidify at a lower temperature compared to the margarine.
- Margarine: Margarine represents a trans fat. During heat treatment, the margarine will melt and reach a higher temperature more quickly compared to the butter. During cold treatment, the margarine will solidify at a higher temperature compared to butter and oil. When a cis fatty acid is converted to a trans fatty acid, the carbon chain is lengthened. Therefore, solid fats that contain trans fatty acids will have higher melting points. These fats also tend to be more solid at room temperature compared to their cis counterparts (e.g. olive oil, vegetable oil). Newer food technologies have been implemented that reduce or remove the production of trans fatty acids in food products such as margarine; however, it is important to look at the food label to determine if a food contains trans fat (see Fascinating Fats activity in the Interest Approach).
- Oil: Vegetable oil contains unsaturated fat and has a lower melting point. The oil is already liquid at room temperature. Additionally, the oil will not solidify during cold treatment, but rather take on a cloudy appearance. Oils are unsaturated fats, meaning the carbons within the fatty acids are not completely saturated with hydrogen.
- Allow students to work in small groups on the Lipid Language lab sheet to further explore the topic and respond to lab questions.
- Follow-up with a class discussion about sources of different types of dietary fat in real life, and the relevance to overall health and energy balance. See Enriching Activities for ideas on how to further extend this lesson.
Lab 2: Examining Emulsions
Teacher Preparation:
- Consider having your students research the types of lipids (fat), their role in the body, their dietary sources, and the health benefits/consequences of consuming them prior to beginning the lab investigation.
- Prepare materials for each group. To prepare mixtures, each group will need 5/8 cup of vinegar and 5/8 cup of olive oil. Each student group will also need 1 teaspoon of each treatment (ground mustard, honey, paprika, and salt).
- Consider placing materials in organized stations around your classroom. Allow students to visit organized stations to retrieve needed lab materials.
- Timesavers:
- You are encouraged to allow students to measure out their own treatments; but if time is a concern, pre-measuring treatments is recommended.
- Another option is to have each group make 5 jars of the same treatment. Jars can then be exchanged among groups. Another option is to assign four students to rotate around the room delivering each of the four treatments to student groups.
- If you chose to allow students to participate in the optional lesson extension, pre-focus the microscopes to save time.
- If limited sink space is available, place pans or buckets of warm, soapy water and rinse water around the classroom so jars can be sufficiently cleaned for another class. Another option is to consider using disposable containers so that you can easily discard the mixtures after completing the lab.
- Timesavers:
Procedures:
- Consider having your students research common mixtures (e.g. homogeneous, heterogeneous) that can be eaten prior to beginning the lab investigation.
- Distribute lab materials. It is recommended that materials are organized into stations for easier distribution. Students should be arranged in small groups of 4-5. Each group should receive the lab supplies outlined in the Materials section as well as 1 copy of the Examining Emulsions lab sheet.
- If students did not complete Lab 1, ask students to read the Fatty Distinction student handout and complete the "Think About It" questions for this lab investigation.
- Before beginning the lab investigation:
- Require students to wash their hands.
- Emphasize the importance of practicing good food safety behaviors by not consuming substances used as part of the lab investigation.
- Note that students should be allowed to taste their final product as part of the lab investigation.
- Launch the student lab investigation by asking students to make a prediction about the emulsification abilities of common household ingredients.
- Show students the provided video lab demonstration, Food Explorations Lab II: Examining Emulsions. The video will help students understand the procedures and visual observations. Students should observe the following:
- The ground mustard should serve to emulsify the vinegar and oil. Ground mustard serves as an emulsifier because it contains protein, carbohydrate, and lipid in its seed. The protein and carbohydrate molecules are hydrophilic (water soluble), while the lipid molecules are hydrophobic (fat soluble). This unique combination of molecules enables ground mustard to aid in the creation of a homogenous vinegar and oil mixture. Egg yolks are another common food-based emulsifier.
- Honey, paprika, and salt are not emulsifiers. None of these ingredients will prevent the oil and vinegar from separating (heterogeneous mixtures). However, when the students combine the mixtures, the vinaigrette should create a homogeneous mixture due to the presence of the ground mustard.
- Allow students to work in small groups on the Examining Emulsions lab sheet to further explore the topic and respond to lab questions.
Optional Lab Extension:
- Launch the lab extension by allowing students time to observe a fat emulsion under the microscope.
- Follow-up with a class discussion about unsaturated dietary fats (e.g. olive oil) and their health benefits. See Enriching Activities for ideas on how to further extend this lesson.
Elaborate
-
Make a lava lamp! Use an empty plastic liter bottle. Add water dyed with food coloring and oil.
-
Discuss the insoluble properties of lipids and describe this characteristic’s importance to cell membrane function (e.g. phospholipids).
-
Build a 3D model of the cell membrane.
Evaluate
After conducting these activities, review and summarize the following key concepts:
- Fat in our diet comes in three forms: saturated, unsaturated, and trans (hydrogenated).
- A melting point is when a solid fat turns to a liquid. Solidification or freezing is when a liquid turns to a sold.
- An emulsion is a mixture of two immiscible liquids like water and oil or vinegar and oil.
Sources
- https://authoritynutrition.com/foods/butter/
- http://www.mayoclinic.org/healthy-lifestyle/nutrition-and-healthy-eating/expert-answers/butter-vs-margarine/faq-20058152
Acknowledgements
This lesson was partnered with East Carolina University. The FoodMASTER program was supported by the Science Education Partnership Award (SEPA) which is funded from the National Center for Research Resources, a component of the National Institutes of Health.
- Primary Authors:
- Virginia Stage, PhD, RDN, LDN
- Mary White
- Ashley Roseno, MAEd, MS, RDN, LDN
- Melani W. Duffrin, PhD, RDN, LDN
- Graphic Design: Cara Cairns Design, LLC
Recommended Companion Resources
Author
Organization
| We welcome your feedback! If you have a question about this lesson or would like to report a broken link, please send us an email at [email protected]. If you have used this lesson and are willing to share your experience, we will provide you with a coupon code for 10% off your next purchase at AgClassroomStore. |
